 The properties of water under conventional conditions are largely known to scientists. But those under unusual cases are rarely revealed. One example is, what happens to the viscosity and elasticity of water confined to two solids in thin layer of nanometer? According to a recent study[1], there may happen a solid-like transition with respect to the rate at which the two solids approach each other, that is, elasticity increases while viscosity decreases.
The properties of water under conventional conditions are largely known to scientists. But those under unusual cases are rarely revealed. One example is, what happens to the viscosity and elasticity of water confined to two solids in thin layer of nanometer? According to a recent study[1], there may happen a solid-like transition with respect to the rate at which the two solids approach each other, that is, elasticity increases while viscosity decreases.[1]Phys. Rev. Lett. 105, 106101 (2010)
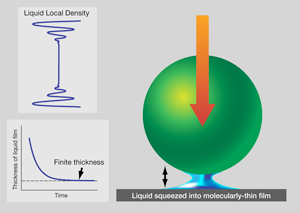
Schematic illustration of a confined fluid. Imagine that a liquid droplet is placed between a ball and a flat surface, and a ball is allowed to fall (right panel) onto it. When the thickness of the liquid is plotted schematically against time after the ball begins to fall, the film thickness remains finite at equilibrium (bottom left panel). This is because fluid tends to layer parallel to the solid surfaces. When the local liquid density is plotted against the distance between the solid boundaries, it shows decaying oscillations with a period of about a molecular dimension (top left panel). When these density waves shown in the bottom panel come sufficiently close to interfere with one another, the liquid can support force at equilibrium.
The fig and caption come from http://physics.aps.org/articles/v3/73
ReplyDelete